ONE PRODUCT FOR TWO INDICATIONS
Y-STRUT® is an implantable medical device indicated for internal fixation of proximal femur of patients at risk of osteoporotic fracture and for impeding pathological fracture. It is composed of 2 cannulas in PEEK (polyetheretherketone) polymer. It is intended to the biomechanical reinforcement of the proximal femur. Y-STRUT® is combined with PMMA bone cement to allow bone anchoring.
FIND OUT MORE
TRAUMATOLOGY INDICATION
Y-STRUT® is indicated for contralateral percutaneous internal fixation of proximal femur, in patient with a low energy pertrochanteric fracture on the first side.
It is implanted during the same anaesthesia than the hip fracture treatment.
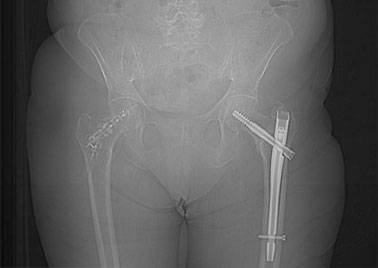
ONCOLOGY INDICATION
Y-STRUT® is indicated for percutaneous internal fixation for impending pathological fracture of proximal femur - act of last resort (ultima ratio).
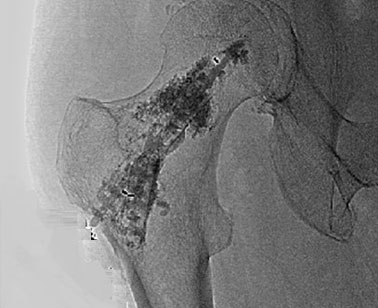 FIND OUT MORE
FIND OUT MORE
MINIMALLY INVASIVE PROCEDURE
Y-STRUT® is implanted by minimally invasive surgery. Only 2 small incisions are performed on the thigh. It is inserted in the proximal femur thanks to an instrumentation specifically developed for a safe implantation.

Y-STRUT® reinforces the biomechanical performance of the proximal femur.
See the article published in Clinical Biomechanics
See the audioslides presentation of the article
CLINICAL STUDIES
TRAUMATOLOGY INDICATION
A prospective clinical study is ongoing.
ONCOLOGY INDICATION
A first clinical study on a 10-patient cohort was performed with one-year follow-up. A post-market observational study is ongoing.
See the article published in the Journal of Orthopaedic Surgery and Research
See the article published in CardioVascular Interventional Radiology